Dioxa-1,3-cyclohexanes
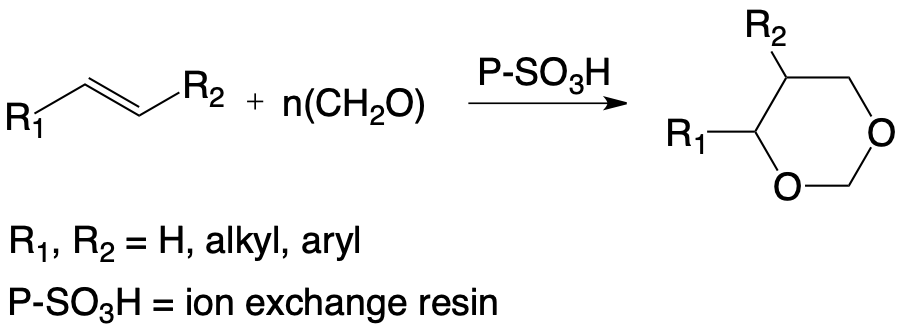
The use of strong acidic ion exchange resins selectively condenses formaldehyde, mainly in the form of paraformaldehyde with various ethylenic substrates. The aromatic alkenes, in particular those derived from biomass, such as isoeugenol or isosafrole, are thus quantitatively converted into 1,3-dioxa-cyclohexane, corresponding to a significant stereoselectivity of the reaction in favor of the trans isomer.
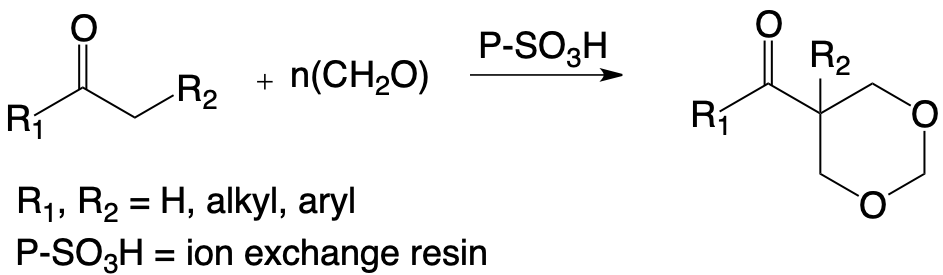
The exploitation of the keto-enol equilibrium of ketones having a methylene group in alpha of the carbonyl makes it possible to obtain, under excellent conditions of yield and selectivity of the 1,3-dioxa-cyclohexanes, certainly different from those obtained from alkenes, but susceptible to applications in many fields.
The stabilized enol function of the phenols, such as vanillin, also reacts under these reaction conditions with the polymerized formaldehyde to give benzo (4H) 1,3-dioxanes with yields and selectivity that are much higher than those observed in a homogeneous medium.
Conformational analysis (1H NMR, 13C, crystallography, etc.) makes it possible to specify the fine structure of these molecules and contributes to the establishment of the different reaction mechanisms.
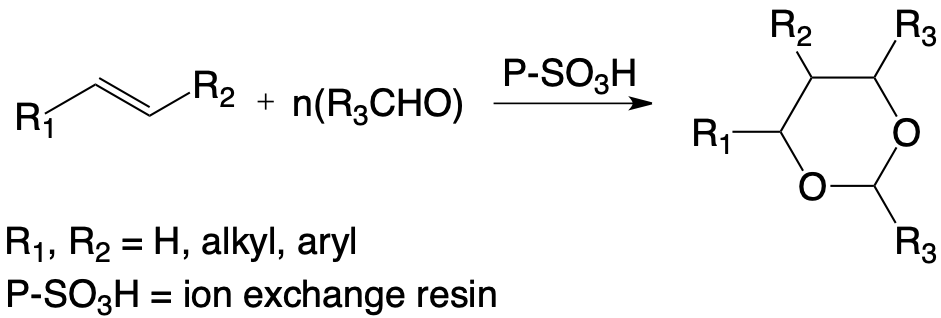
Obtaining new dioxane compounds with aldehydes other than formaldehyde inaccessible by another route of synthesis has therefore required the determination of optimal conditions of condensation depending essentially on :
- the hydration rate of the reaction medium,
- the physical and chemical nature of the macromolecular network of the ion exchanger,
- the nature of the organic solvent.
The discussion of the reaction mechanisms has revealed new stereoisomerization reactions which appear between the new different stereoisomeric dioxa-1,3 cyclohexanes obtained from the same alkene.
Phosphorus and nitrogen macrocycles / Saturated triazines
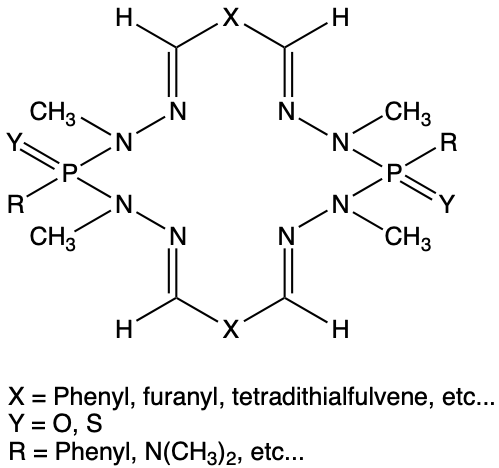
This was the first macrocycle synthesis carried out without an ionic catalyst by bringing into contact variously functionalized dialdehydes with phosphodihydrazides. The yield is quantitative. The 2,5-furan dicarboxaldehyde and the phenolic dialdehydes whose synthesis is reported in the following lines are at the origin of the main macrocycles synthesized. The first realized RX structure shows a rare structural arrangement : “butterfly”. These molecules can be bridged, reduced or oxidized to a new class of selective complexing agents.
This original technique has been applied to manufacture from furan di and tetra-amines manufactured for the occasion of new nitrogenous macrocycles which are remarkable selective complexing agents of the silver cation.
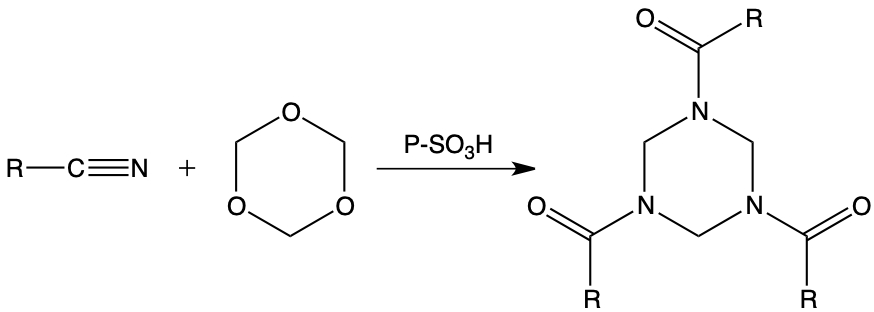
Another interesting application of this particular reactivity of the polymerized formaldehyde system / cationic ion exchangers, in a weakly hydrated organic medium, concerns saturated and unsaturated nitriles which, condensed with paraformaldehyde in the presence of ion exchange resins, lead to the corresponding saturated triazines with remarkable yields.
The structural study of these compounds is carried out in parallel with the establishment of reaction schemes compatible with the results of the reaction kinetics analysis.
Furanic heterocycles
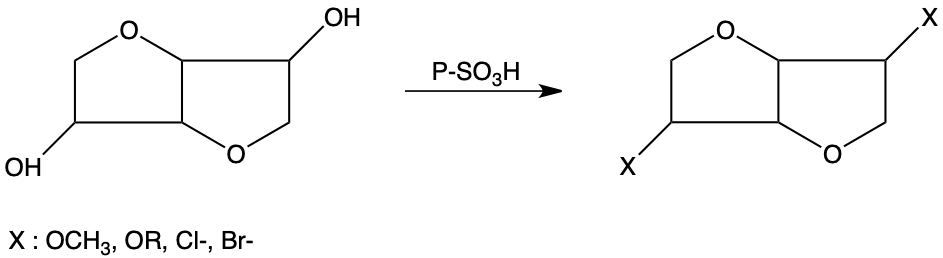
The halogenation and alkylation of isosorbide and isomannide selectively leads to :
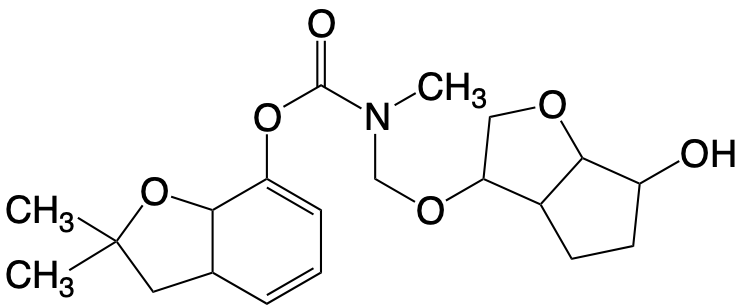
Hydroxymethylation with formaldehyde allows the synthesis of variously substituted carbamates